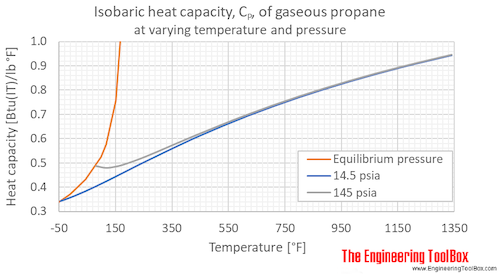
The specific heat of air at constant pressure is 1.05kj//kg K and the specific heat of air at - YouTube

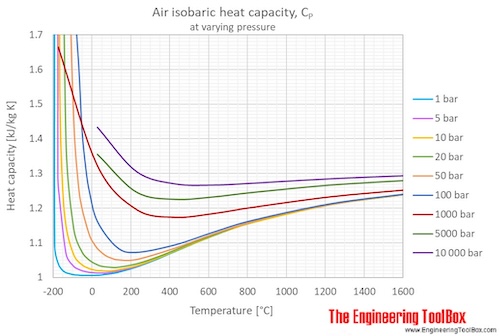
3: Specific heat at constant pressure vs. temperature, for air and CO 2 . | Download Scientific Diagram
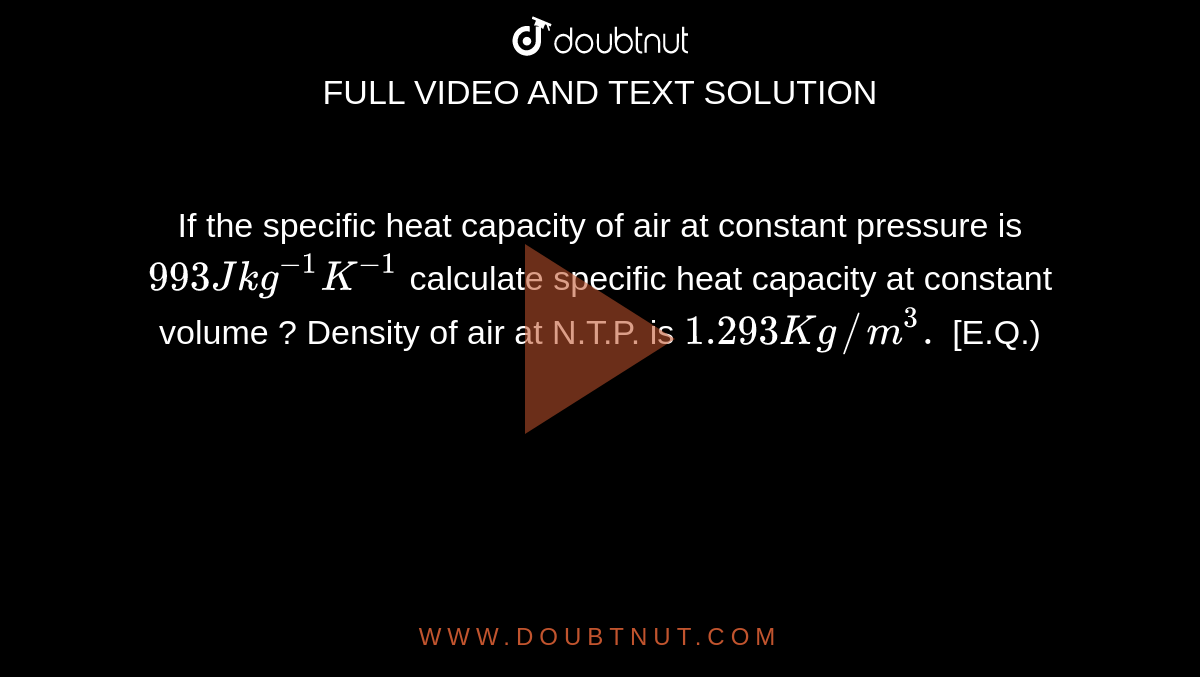
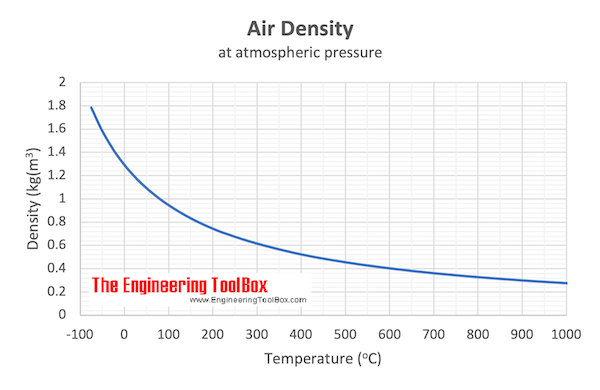
If the specific heat capacity of air at constant pressure is 993 J kg ^(-1) K ^(-1) calculate specific heat capacity at constant volume ? Density of air at N.T.P. is 1.293 Kg //m ^(3). [E.Q.)

1: Specific heat at constant pressure vs. temperature for air and CO 2 | Download Scientific Diagram

SOLVED: of '390 K The density of A hot air balloon contains 850 cm' ofair at an assumed constant temperature the air at this temperature is 0.903 m J heat of the

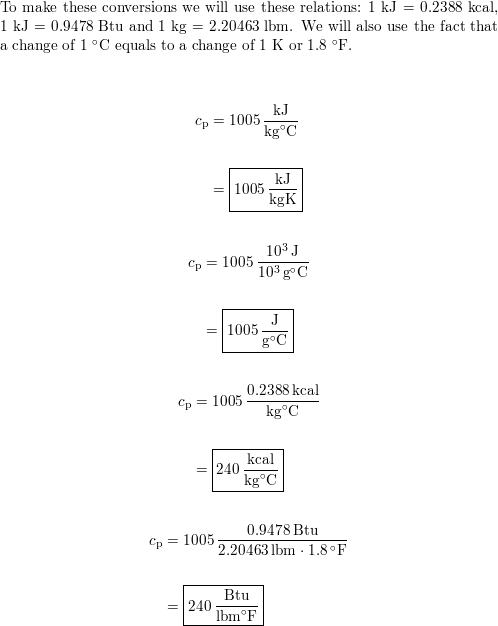
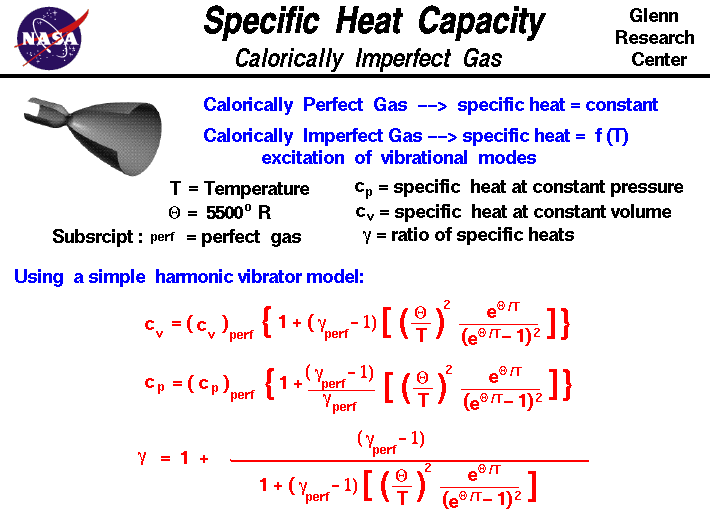
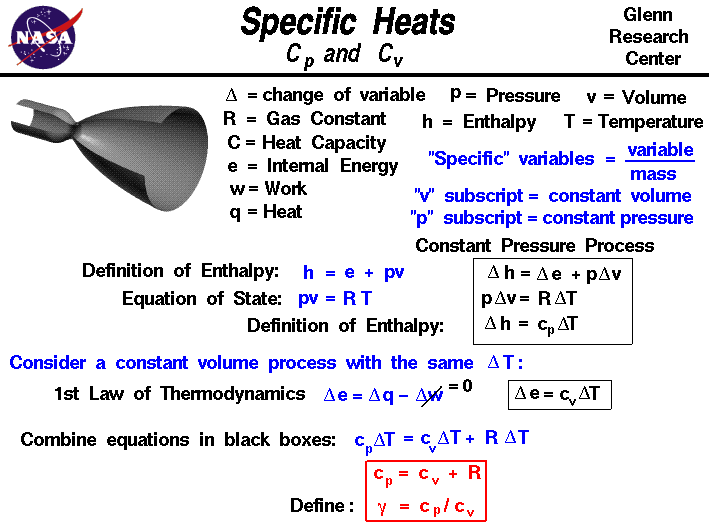
The specific heat of air at constant pressure is 1.005 kJ/kg K and the specific heat of air at constant volume is 0.718 kJ/kg K .Find the specific gas constant.

Estimation of air specific heat ratio at elevated pressures using simple predictive tool - ScienceDirect