Evaluation of reagents used to coat the hollow-fiber bioreactor membrane of the Quantum® Cell Expansion System for the culture of human mesenchymal stem cells - ScienceDirect

Automated Quantum Cell Expansion System (left) showing the graphic user... | Download Scientific Diagram

Large-scale production of lentiviral vector in a closed system hollow fiber bioreactor: Molecular Therapy - Methods & Clinical Development

Evaluation of reagents used to coat the hollow-fiber bioreactor membrane of the Quantum® Cell Expansion System for the culture of human mesenchymal stem cells - ScienceDirect

The Quantum System hydraulic layout with the IC loop shown in red and... | Download Scientific Diagram

Characterization and cost–benefit analysis of automated bioreactor‐expanded mesenchymal stem cells for clinical applications - Russell - 2018 - Transfusion - Wiley Online Library

Automated Good Manufacturing Practice–compliant generation of human monocyte-derived dendritic cells from a complete apheresis product using a hollow-fiber bioreactor system overcomes a major hurdle in the manufacture of dendritic cells for cancer
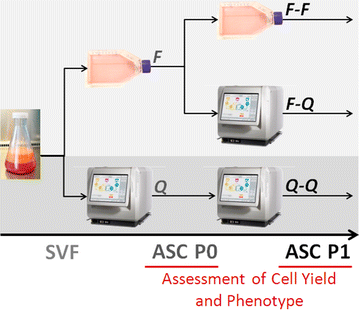
Culture expansion of adipose derived stromal cells. A closed automated Quantum Cell Expansion System compared with manual flask-based culture | Journal of Translational Medicine | Full Text

Figure 1 from Efficient manufacturing of therapeutic mesenchymal stromal cells with the use of the Quantum Cell Expansion System. | Semantic Scholar

GMP-Compliant Expansion of Clinical-Grade Human Mesenchymal Stromal/Stem Cells Using a Closed Hollow Fiber Bioreactor | SpringerLink