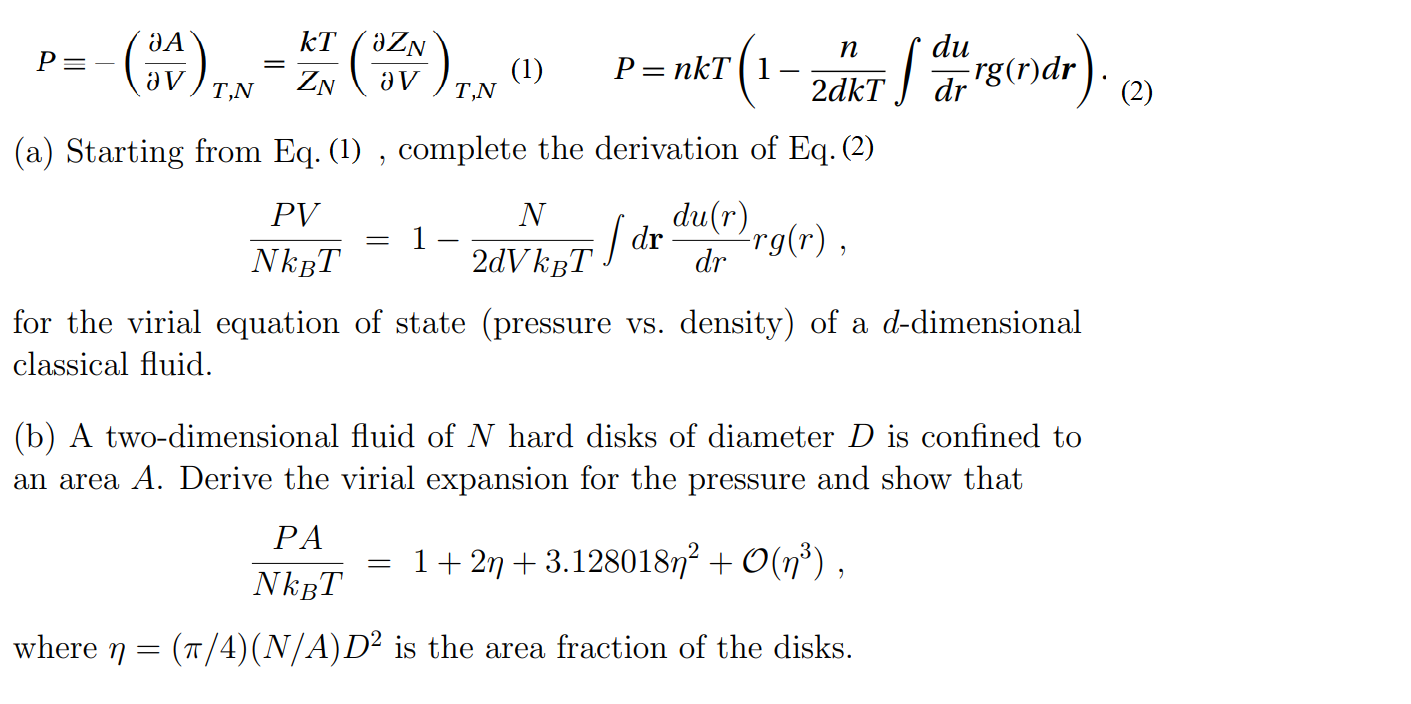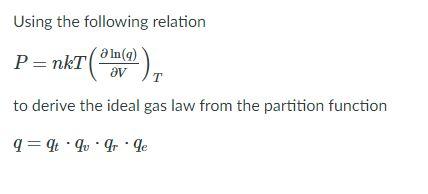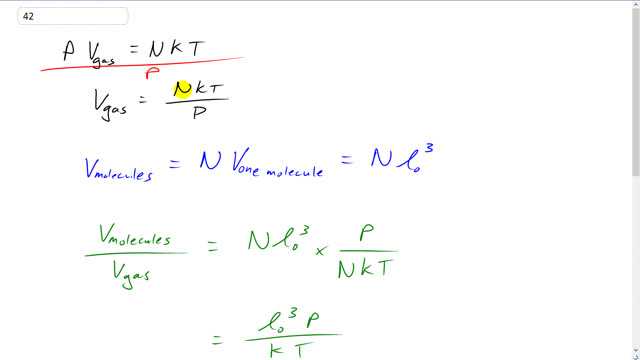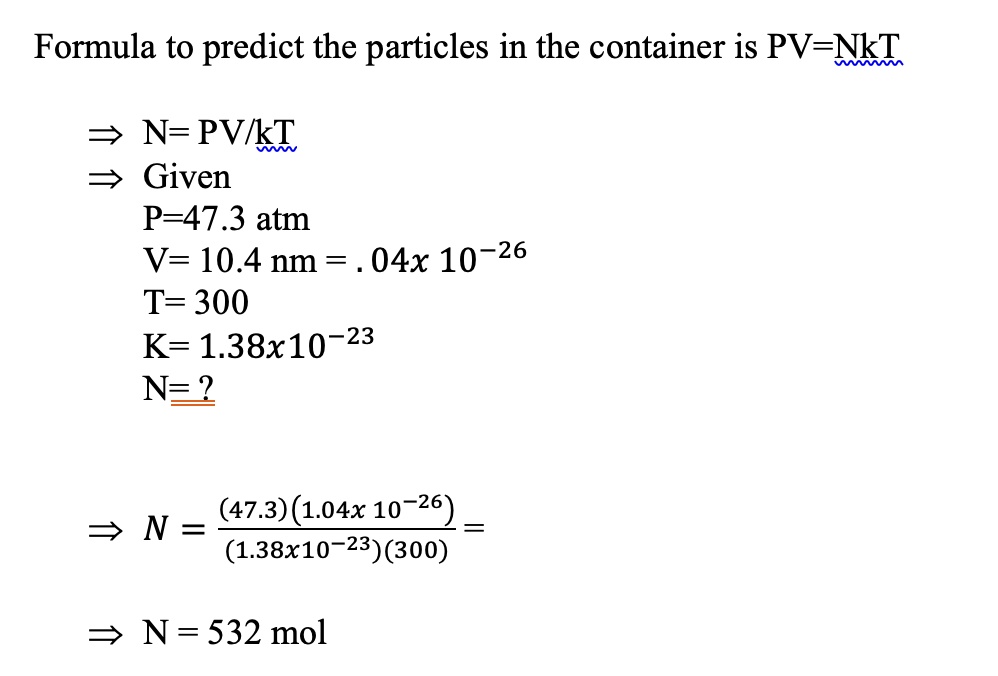
SOLVED: Formula to predict the particles in the container is PV-NkT N= PVIkT Given P-47.3 atm V= 10.4 nm = 04x 10-26 T=300 K= 1.38x10-23 N= 2 (47.3)(1.04x 10-26 N = (1.38x10-23)(300) N= 532 mol

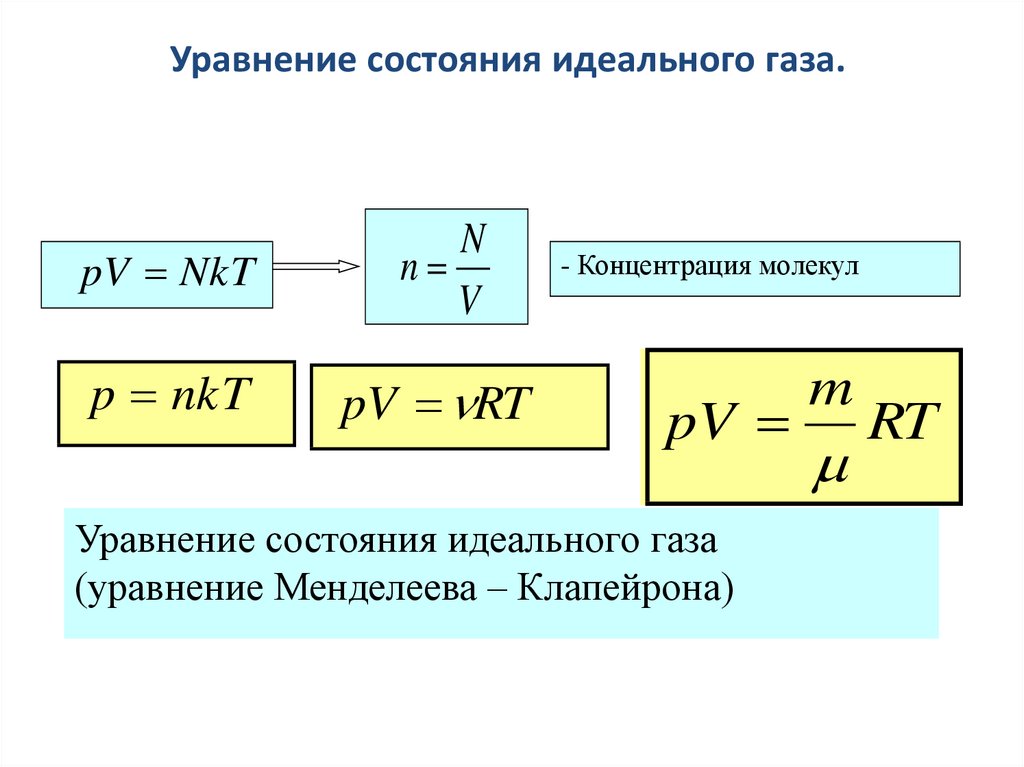
METR February Review State variables: p, ρ, T Pressure Temperature Equation of state: p = NkT/V = ρ R d T Virtual temperature T v = T (1. - ppt download

SOLVED: Recall the integrals: f FIn x + Constant OR dx =In X2 In X1 = In- Use this expression to calculate Wby dV when the ideal gas law pV = NkT
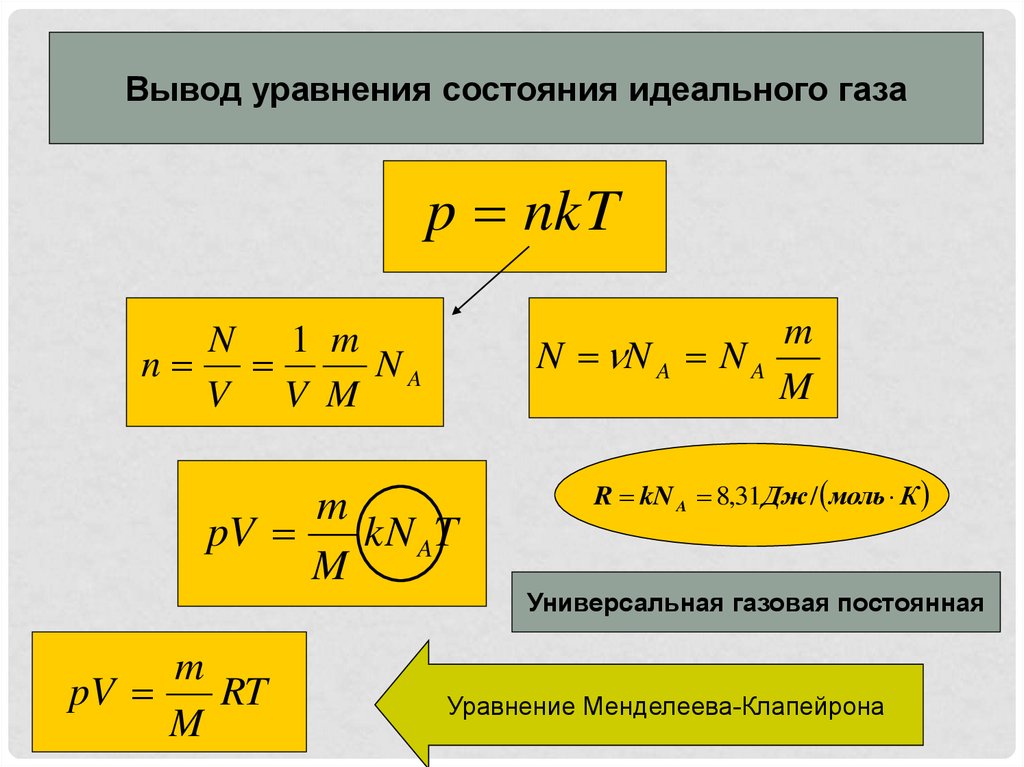
NkT PV = nRT PV = Pa pressure P = m volume V = moles n particles N # # = = kelvin e temperatur T = KJ x Const Boltzmann k / 1038

Summary plot of an overall comparison of NKT (CD3 ϩ CD16/56 ϩ ; p Ͻ... | Download Scientific Diagram