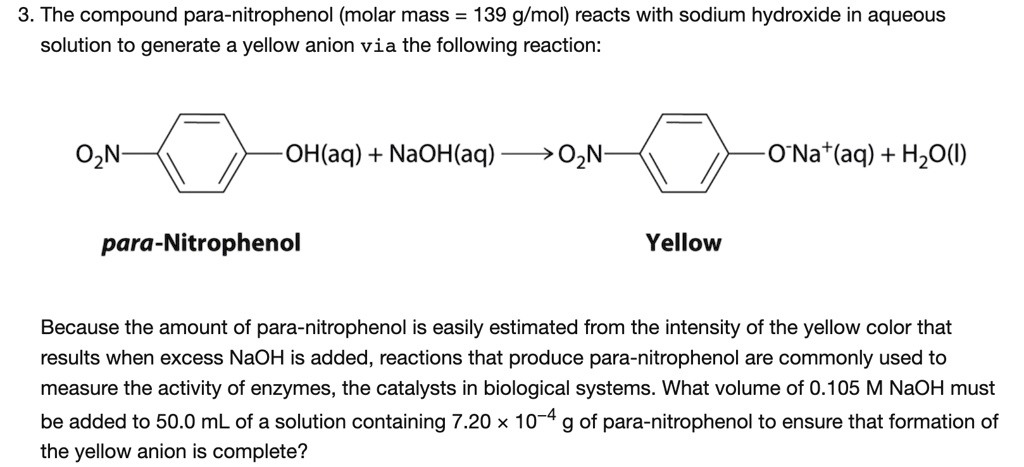
SOLVED: 3. The compound para-nitrophenol (molar mass = 139 g/mol) reacts with sodium hydroxide in aqueous solution to generate a yellow anion via the following reaction: OzN OH(aq) + NaOH(aq) O2N 0
How should I balance this equation P + NaOH + H2O---------> PH3 + NaH2PO2 by ion electron method? - Quora

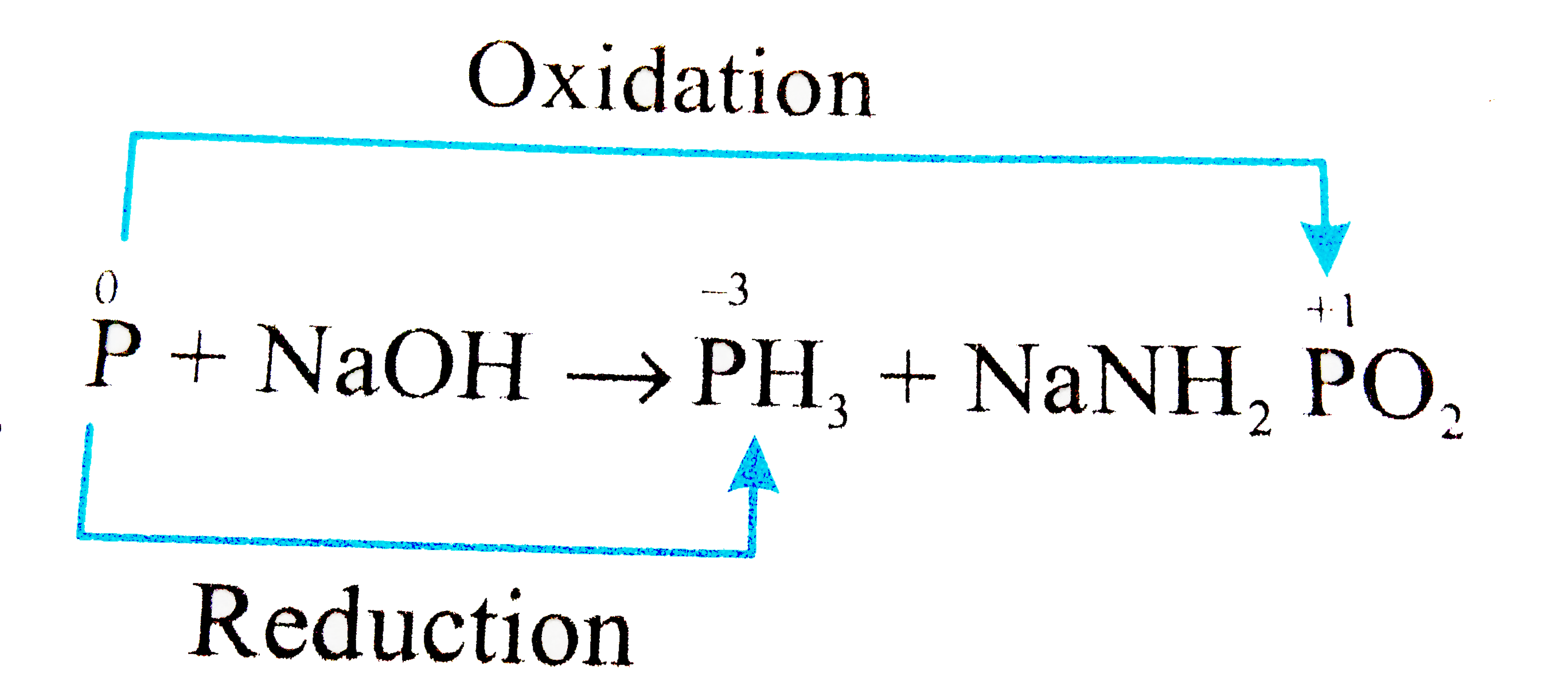
The reaction of white phosphorus with aqueous NaOH gives phosphine along with another phosphorus containing compound. The reaction type, the oxidation states of phosphorus in phosphine and the other product are respectively:

Immobilization and characterization of Fe(0) catalyst on NaOH-treated coal fly ash for catalytic reduction of p-nitrophenol - ScienceDirect

Write the product formed when p-nitrochlorobenzene is heated with aqueous naoh at 443k followed by - Brainly.in

Unexpected Complexity in the Products Arising from NaOH-, Heat-, Amine-, and Glycosylase-Induced Strand Cleavage at an Abasic Site in DNA | Chemical Research in Toxicology

The reaction of white phosphorus with aqueous NaOH gives phosphine along with another phosphorus containing compound. The reaction type, the oxidation states of phosphorus in phosphine and the other product are respectively:

White phosphorus on reaction with concentrated NaOH solution in an inert atmosphere of CO2 gives phosphine and compound (X) . (X) on acidification with HCl gives compound (Y) . The basicity of

Write an equation for the reaction of p-bromobenzaldeyde with HCN (NaOH as the catalyst). | Homework.Study.com
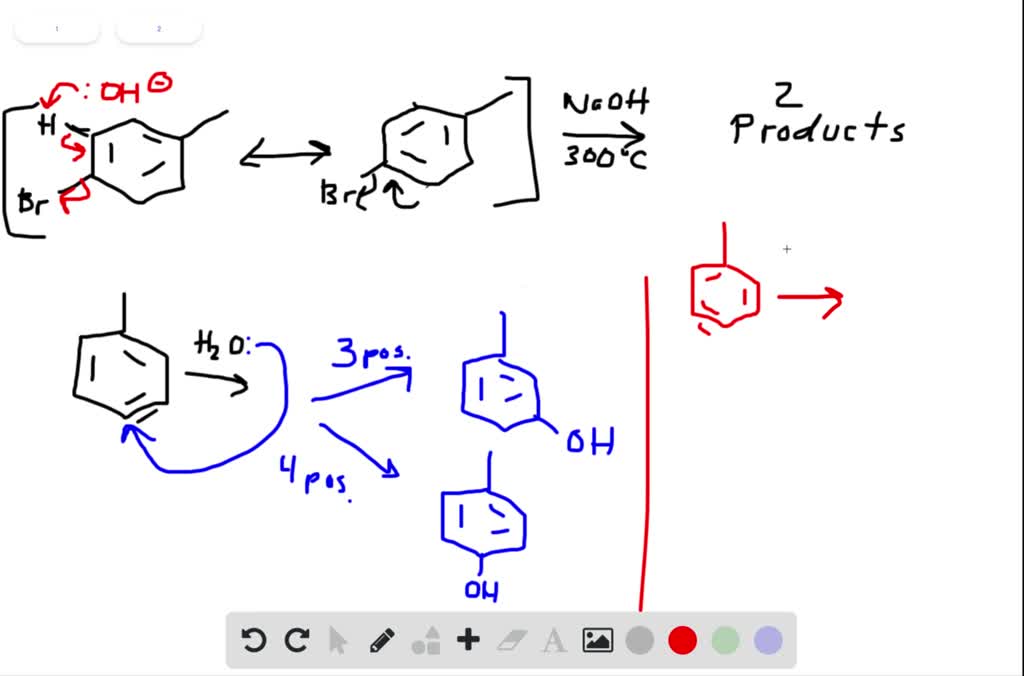
SOLVED:Treatment of p -bromotoluene with NaOH at 300^∘ C yields a mixture of two products, but treatment of m -bromotoluene with NaOH yields a mixture of three products. Explain.

Write the Product Formed When P-nitro Chlorobenzene is Heated with Aqueous Naoh at 443k Followed by Acidification? - Chemistry | Shaalaa.com
![C/P Section Bank #14] I understand why we use NaOH in the first step. However, I don't understand why choosing to use diethyl ether over HCl. Can't the HCl react with the C/P Section Bank #14] I understand why we use NaOH in the first step. However, I don't understand why choosing to use diethyl ether over HCl. Can't the HCl react with the](https://preview.redd.it/lr1b4v0aahk11.jpg?auto=webp&s=643d5163549702152d86a419e1ff1f1f3717feee)
C/P Section Bank #14] I understand why we use NaOH in the first step. However, I don't understand why choosing to use diethyl ether over HCl. Can't the HCl react with the
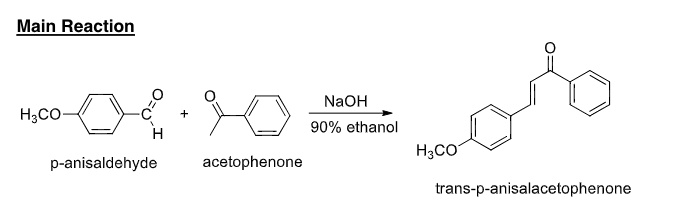
SOLVED: Main Reaction NaOH 90% ethanol HzCO acetophenone trans-p-anisalacetophenone HzCo p-anisaldehyde


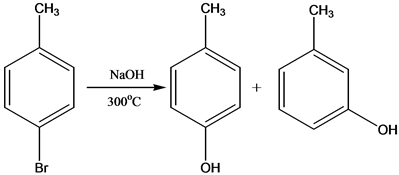



![ANSWERED] Consider the following three solutions of... - Physical Chemistry ANSWERED] Consider the following three solutions of... - Physical Chemistry](https://media.kunduz.com/media/sug-question/raw/59737718-1659709481.9039986.jpeg)